What are the most common types of radioactive decay? How can we protect ourselves against the harmful effects of the resulting radiation?
Depending on the type of particles or waves that the nucleus releases to become stable, there are various kinds of radioactive decay leading to ionizing radiation. The most common types are alpha particles, beta particles, gamma rays and neutrons.
Alpha radiation
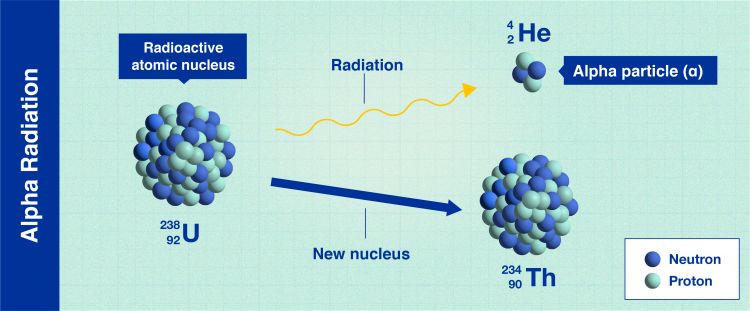
Alpha decay (Infographic: A. Vargas/IAEA).
In alpha radiation, the decaying nuclei release heavy, positively charged particles in order to become more stable. These particles cannot penetrate our skin to cause harm and can often be stopped by using even a single sheet of paper.
However, if alpha-emitting materials are taken into the body by breathing, eating, or drinking, they can expose internal tissues directly and may, therefore, damage health.
Americium-241 is an example of an atom that decays via alpha particles, and it is used in smoke detectors across the world.
Beta radiation
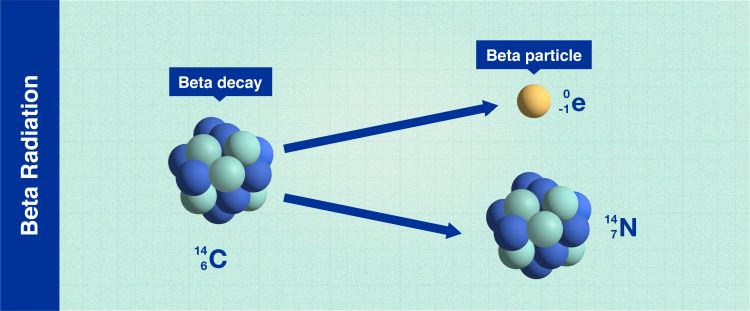
Beta decay (Infographic: A. Vargas/IAEA).
In beta radiation, the nuclei release smaller particles (electrons) that are more penetrating than alpha particles and can pass through e.g., 1-2 centimetres of water, depending on their energy. In general, a sheet of aluminium a few millimetres thick can stop beta radiation.
Some of the unstable atoms that emit beta radiation include hydrogen-3 (tritium) and carbon-14. Tritium is used, among others, in emergency lights to for instance mark exits in the dark. This is because the beta radiation from tritium cause phosphor material to glow when the radiation interacts, without electricity. Carbon-14 is used to, for example, date objects from the past.
Gamma rays
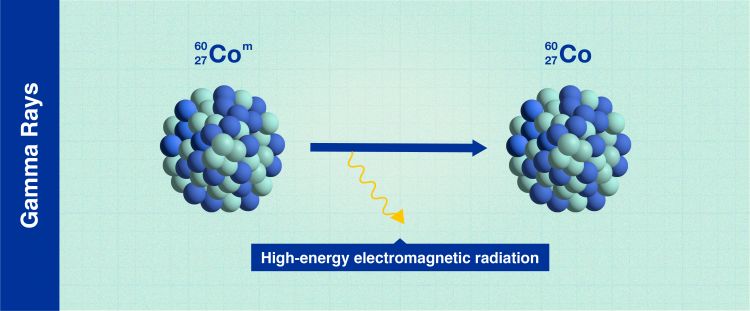
Gamma rays (Infographic: A. Vargas/IAEA).
Gamma rays, which have various applications, such as cancer treatment, are electromagnetic radiation, similar to X-rays. Some gamma rays pass right through the human body without causing harm, while others are absorbed by the body and may cause damage. The intensity of gamma rays can be reduced to levels that pose less risk by thick walls of concrete or lead. This is why the walls of radiotherapy treatment rooms in hospitals for cancer patients are so thick.
Neutrons
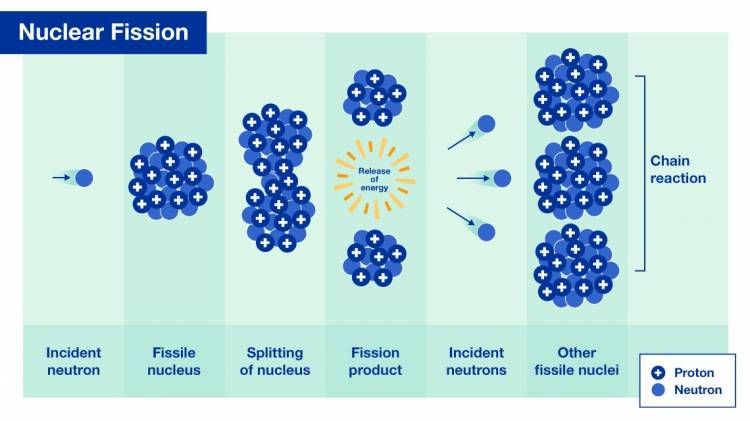
Nuclear fission inside a nuclear reactor is an example of a radioactive chain reaction sustained by neutrons (Graphic: A. Vargas/IAEA).
Neutrons are relatively massive particles that are one of the primary constituents of the nucleus. They are uncharged and therefore do not produce ionization directly. But their interaction with the atoms of matter can give rise to alpha-, beta-, gamma- or X-rays, which then result in ionization. Neutrons are penetrating and can be stopped only by thick masses of concrete, water or paraffin.
Neutrons can be produced in a number of ways, for example in nuclear reactors or in nuclear reactions initiated by high-energy particles in accelerator beams. Neutrons can represent a significant source of indirectly ionizing radiation.
Post time: Nov-11-2022

